 |
 |
C000 General Information
C020 Restricted or Nonmailable Articles and Substances
C023 Hazardous Materials
Summary
C023 describes the general standards, restrictions, and prohibitions that apply to the mailability of hazardous materials.
1.0 General
1.1Definitions
The following definitions apply:
a. [6-12-03] Hazardous material is any article or substance designated by the U.S. Department of Transportation (DOT) as being capable of posing an unreasonable risk to health, safety, and property during transportation. In international commerce, hazardous materials are known as “dangerous goods.”
b. [6-12-03] Limited quantity is the maximum amount of a specific hazardous material that is exempted from the labeling or packaging requirements in 49 CFR. Not every hazardous material is eligible to be shipped as a limited quantity. Almost all limited quantity materials are nonmailable.
c. [6-12-03] ORM-D (Other Regulated Material) material is a limited quantity of a hazardous material that presents a limited hazard during transportation due to its form, quantity, and packaging. In almost all instances, the proper shipping name for an ORM-D material is consumer commodity. Not all hazardous material permitted to be shipped as a limited quantity can qualify as an ORM-D material. ORM-D materials having the proper shipping name of “consumer commodity” are mailable subject to USPS quantity and packaging standards.
d. Consumer commodity is a hazardous material that is packaged and distributed in a quantity and form intended or suitable for retail sale and designed for consumption by individuals for their personal care or household use purposes. This term can also include certain drugs or medicines. Not all hazardous material permitted to be shipped as a limited quantity can qualify as a consumer commodity.
e. [6-12-03] Air transportation requirements, for the purposes of C023 only, apply to all mailable hazardous materials sent at the First-Class Mail, Priority Mail, or Express Mail rates. All mailable hazardous materials sent at those rates must meet the requirements that apply to air transportation. Mailable hazardous materials sent at any of those rates may or may not be transported via air depending on the distance between the point of origination and the point of destination, and the ability of the USPS to obtain an air carrier between those points.
f. [6-12-03] Surface transportation requirements, for the purposes of C023 only, apply to all mailable hazardous materials sent at the Standard Mail or Package Services rates. All mailable hazardous materials sent at the Standard Mail or Package Services rates must meet the requirements that apply to surface transportation.
g. Primary receptacle is the container (e.g., tube, vial, bottle) that holds the hazardous material.
h. [6-12-03] Secondary container is the packaging component into which the primary receptacle(s) and any required absorbent and cushioning material is securely placed. The packaging of certain mailable hazardous materials does not require the use of a secondary container.
i. [6-12-03] Outer shipping container is the exterior packaging component into which a primary receptacle, along with any required absorbent and cushioning material, and the secondary container (if required) are securely placed. The outer shipping container bears the addressing information along with all required markings.
1.2U.S. Department of Transportation
[6-12-03] The U.S. Department of Transportation (DOT) regulates the surface and air carriage of hazardous materials within the United States via any means of transportation. The DOT regulations for the transport of hazardous materials are codified in Title 49, Code of Federal Regulations (49 CFR) 100–185. USPS mailing standards for hazardous materials generally adhere to 49 CFR, but also include many additional limitations and prohibitions.
1.3USPS Standards
[6-12-03] The USPS standards generally restrict the mailing of hazardous materials to ORM-D materials with the proper shipping name of “consumer commodity” that meet USPS quantity limitations and packaging requirements. The few non-ORM-D materials permitted to be mailed are subject to the standards in C023. Detailed information on the mailability of specific hazardous materials is contained in Publication 52, Hazardous, Restricted, and Perishable Mail.
1.4Hazard Class
Every hazardous material is assigned to one of nine hazard classes identified in 49 CFR 172.101 and 173. Some hazard classes are further separated into divisions based on their physical or chemical properties. For postal purposes, Exhibit 1.4 generally summarizes the mailability of hazardous materials by hazard class.
Exhibit 1.4DOT Hazard Classes and Mailability Summary
| Class | Hazard Class Name and Division | Domestic Mail Air Transportation |
Domestic Mail Surface Transportation |
International Mail |
|---|---|---|---|---|
| 1 | Explosives Division 1.1 Mass Explosive Hazard Division 1.2 Projection Hazard Division 1.3 Fire Hazard and/or Minor Blast/Minor Projection Hazard Division 1.4 Minor Blast Hazard Division 1.5 Very Insensitive With Mass Explosion Hazard Division 1.6 Extremely Insensitive With No Mass Explosion Hazard |
Prohibited | Prohibited except with written permission as allowed in 2.2 | Prohibited |
| 2 | Gases Division 2.1 Flammable Gases Division 2.2 Nonflammable, Nontoxic Gases Division 2.3 Toxic Gases |
Division 2.1 and 2.3: Prohibited. Division 2.2: Only ORM-D material per 3.3 |
Divisions 2.1, 2.2: Only ORM-D
material per 3.3. Division 2.3: Prohibited |
Prohibited |
| 3 | Flammable and Combustible Liquids | Flammable liquids: Prohibited. Combustibles: Only ORM-D material per 4.3 |
Flammable liquids: Only ORM-D
material per 4.2. Combustibles: Only ORM-D material per 4.3 |
Prohibited |
| 4 | Flammable Solids Division 4.1 Flammable Solids Division 4.2 Spontaneously Combustible Division 4.3 Dangerous When Wet |
Prohibited | Only ORM-D material per 5.2 | Prohibited |
| 5 | Oxidizing Substances, Organic
Peroxides Division 5.1 Oxidizing Substances Division 5.2 Organic Peroxides |
Only ORM-D material per 6.2 | Only ORM-D material per 6.2 | Prohibited |
| 6 | Toxic Substances and Infectious
Substances Division 6.1 Toxic Substances Division 6.2 Infectious Substances |
Division 6.1: Only ORM-D
material per 7.2. Division 6.2: Only per 8.0 |
Division 6.1: Only ORM-D
material per 7.2. Division 6.2: Only per 8.0 |
Division 6.1: Prohibited. Division 6.2: Only mailable per IMM 135 |
| 7 | Radioactive Materials | Prohibited | Only in limits per 9.0 and Publication 52 | Only mailable in limits per IMM 135 |
| 8 | Corrosives | Only ORM-D material per 10.2 | Only ORM-D material per 10.2 | Prohibited |
| 9 | Miscellaneous Hazardous Materials | Only ORM-D material per 11.0 | Only ORM-D material per 11.0 | Prohibited, except magnetized materials per IMM 136 |
1.5Mailer Responsibility
Full responsibility rests with the mailer to comply with all postal and nonpostal laws and regulations regarding the mailing of hazardous materials. Anyone who mails, or causes to be mailed, a nonmailable or improperly packaged hazardous material can be subject to legal penalties, including but not limited to those specified in 18 USC.
1.6Mailability Rulings
[6-12-03] Generally, the acceptability for mailing chemicals and other types of hazardous materials depends on container fluid/vapor capacities, the ability of the complete mailpiece to contain the material, and the method of absorbing and containing the product in case of accidental leakage of the primary receptacle. To determine mailability of a specific material, a mailer must submit a material safety data sheet (MSDS) and the following information to the appropriate rates and classification service center (RCSC):
a. Name of material, hazard class, and assigned United Nations (UN) or North America (NA) identification number.
b. Chemical composition by percentage of ingredient.
c. Flashpoint.
d. Toxic properties.
e. Irritant action when inhaled, swallowed, or contacted by eyes or skin.
f. Special precautions necessary to permit handling without harm to USPS employees or damage to property or other mail.
g. Explanation of warning labels and shipping papers required by state or federal regulations.
h. Proposed packaging method, including the addressing and required markings.
i. Proposed number of pieces to be mailed, class of mail, and post office(s) of mailing.
1.7Warning Labels
[6-12-03] With few exceptions as noted in these standards, most hazardous materials acceptable for mailing fall within the Other Regulated Materials (ORM-D) regulations of CFR 49 173.144, which do not require DOT hazard class warning labels. Except for Division 6.2 materials under 8.5 and dry ice under 11.4, any hazardous material bearing or required to bear a DOT hazard class warning label under the requirements in 49 CFR is prohibited from mailing. Mailable ORM-D material must be marked as required in 1.8. Mailable hazardous material must bear DOT handling labels (e.g., orientation arrows, magnetized materials) when applicable.
1.8Package Markings
[6-12-03] Each mailpiece containing a mailable hazardous material must be plainly and durably marked on the address side with the required shipping name and UN identification number. The UN identification number is not required on a mailpiece that contains an ORM-D material. A mailable ORM-D material must be marked on the address side with “ORM-D” or “ORM-D AIR,” as applicable, immediately following or below the proper shipping name. The proper shipping name for a mailable ORM-D material is “consumer commodity.” The designation “ORM-D” or “ORM-D AIR”, as required, must be placed within a rectangle that is approximately 6.3 mm (1/4 inch) larger on each side than the designation. Mailable ORM-D materials sent as Standard Mail or Package Services must also be marked on the address side as “Surface Only” or “Surface Mail Only.”
1.9Shipping Papers
[6-12-03] A shipper’s declaration for dangerous goods (i.e., shipping paper) prepared under 49 CFR 172.200 through 172.205 is required for certain types of hazardous materials when mailed. The shipping paper must be completed and signed in triplicate by the mailer. It must be affixed to the outside of the mailpiece within an envelope or similar carrier that can be easily opened and resealed to allow viewing of the document. Shipping papers are required as follows:
a. Air transportation requirements. Except for nonregulated materials sent under 8.3 or 8.10 and diagnostic specimens sent under 8.6, mailpieces containing mailable hazardous materials sent at the First-Class Mail, Priority Mail, or Express Mail rates must include a shipping paper.
b. Surface transportation requirements. Except for nonregulated materials sent under 8.3 or 8.10 and mailable ORM-D materials, mailpieces containing mailable hazardous materials sent at the Standard Mail or Package Services rates must include a shipping paper.
1.10Air Transportation Prohibitions
[6-12-03] All mailable hazardous materials sent at the First-Class Mail, Priority Mail, or Express Mail rates must meet the requirements for air transportation. The following types of hazardous materials that are prohibited from carriage on air transportation must not be sent at the First-Class Mail, Priority Mail, or Express Mail rates:
a. Anything susceptible to damage or that can become harmful because of changes in temperature or atmospheric pressures unless protected against the effects of such changes.
b. Magnetic materials that have a field strength sufficient to cause a compass deviation at a distance of 15 feet (4.6 meters) or more from any point on the outer packaging.
c. Flammable materials (gases, liquids, and solids).
d. Radioactive materials.
e. Materials excluded from air shipment by DOT regulations (49 CFR 100-185) or of the applicable state (country) or air carrier operator variations. Certain restricted articles, as described in 49 CFR 100-185 and the operator variations of the air carriers, may be accepted for air transportation if properly packaged. These articles must be labeled and bear a shipper's declaration in triplicate, as required by 49 CFR 172.204, or must be marked according to the air carrier's operator variations. Refer to the technical instruction of the International Civil Aviation Organization (ICAO) for air carrier operator variations.
2.0 Explosives (Hazard Class 1)
2.1Definition
An explosive is any substance, article, or device that is designed to function by explosion (i.e., an extremely rapid release of gas and heat) or that, by chemical reaction within itself, is able to function in a similar manner even if not designed to function by explosion, unless the substance or article is otherwise classed under the provisions in 49 CFR. Hazard class 1 has six divisions as shown in Exhibit 1.4. No further explanation of the six divisions is provided in these standards because explosives are prohibited in the mail except as permitted in 2.2.
2.2Mailability
[6-12-03] Explosives are prohibited in international mail. Explosives are prohibited in the domestic mail via air transportation. For domestic surface transportation, explosives are prohibited except for certain Division 1.4S toy propellant devices and safety fuses specifically approved by the manager of Mailing Standards (see G043 for address) before mailing. A mailable explosive must meet the packaging and marking requirements provided with the Manager’s approval. A shipping paper is required.
3.0 Gases (Hazard Class 2)
3.1Definition
Hazard class 2 consists of three divisions:
a. Division 2.1, Flammable Gases. A material that is a gas at 68°F (20°C) or less and 14.7 psi (101.3 kPa) of pressure. Flammable gases also include materials that have a boiling point of 68°F (20°C) or less at 14.7 psi (101.3 kPa) and that are ignitable at 14.7 psi (101.3 kPa) when in a mixture of 13% or less by volume with air or that have a flammable range at 14.7 psi (101.3 kPa) with air of at least 12% regardless of the lower limit. These conditions must be established in accordance with ASTM E681-85, Standard Test Method for Concentration Limits of Flammability of Chemicals, or other approved equivalent method. The flammability of aerosols must be determined using the tests specified in 49 CFR 173.306(i).
b. Division 2.2, Nonflammable, Nontoxic Gases. A material that does not meet the definition of Division 2.1 or 2.3 and exerts in its packaging an absolute pressure of 40.6 psia (280 kPa) or greater at 68°F (20°C).
c. Division 2.3, Toxic Gases. A material that is poisonous by inhalation and is a gas at 68°F (20°C) or less and a pressure of 14.7 psi (101.3 kPa) or a material that has a boiling point of 68°F (20°C) or less at 14.7 psi (101.3 kPa).
3.2Mailability
Gases are prohibited in international mail. Toxic gases in Division 2.3 are prohibited in domestic mail. Flammable gases in Division 2.1 are prohibited in domestic mail via air transportation, but are permitted via surface transportation if the material can qualify as an ORM-D material and meet the standards in 3.3 and 3.4. Nonflammable gases in Division 2.2 are generally permitted in the domestic mail via air or surface transportation if the material can qualify as an ORM-D material and meet the standards in 3.3 and 3.4.
3.3Container
[6-12-03] An other-than-metal primary receptacle containing a mailable gas may be acceptable if the water capacity of the primary receptacle is 4 fluid ounces (7.22 cubic inches) or less per mailpiece and the primary receptacle meets 49 CFR requirements. Mailable nonflammable and flammable compressed gases are acceptable in metal primary receptacles that have a water capacity up to 33.8 fluid ounces (1 liter or 61.0 cubic inches), depending on their internal pressure. A DOT 2P container must be used as the primary receptacle if the internal pressure is from 140 to 160 psig at 130°F (55°C). A DOT 2Q container must be used as the primary receptacle if the pressure is from 161 to 180 psig at 130°F (55°C). A container with an internal pressure over 180 psig at 130°F (55°C) is prohibited from mailing. Mailable flammable compressed gases are restricted to 33.8 fluid ounces (1 liter) per mailpiece. Mailable nonflammable compressed gases are permitted in individual 33.8 fluid ounce (1 liter) containers that must be securely packed within an outer shipping container. Each mailpiece must not exceed a total weight of 25 pounds.
3.4Marking
[6-12-03] For surface transportation, packages of mailable gases must be clearly marked on the address side with “Surface Only” or “Surface Mail Only” and “ORM-D” immediately following or below the proper shipping name (consumer commodity). For air transportation, packages must be plainly and durably marked on the address side with “ORM-D AIR” immediately following or below the proper shipping name and must also bear a shipper's declaration for dangerous goods.
4.0 Flammable and Combustible Liquids (Hazard Class 3)
4.1Definitions
The terms used in the standards that apply to hazard class 3 are defined as follows:
a. Flammable liquid means a liquid that has a flashpoint of not more than 141°F (60.5°C), or any material in a liquid phase that has a flashpoint at or above100°F (38°C).
b. Combustible liquid means any liquid that does not meet the definition of any other hazard class and has a flashpoint above 141°F (60.5°C) and below 200°F (93°C). Note: A flammable liquid with a flashpoint at or above 100°F (38°C) that does not meet the definition of any other hazard class may be reclassified as a combustible liquid per 49 CFR 173.120(b).
4.2Flammable Liquid Mailability
[6-12-03] Flammable liquid is prohibited in international mail. Flammable liquid with a flashpoint of 20°F (-7°C) or below is prohibited in domestic mail. Other flammable liquid is prohibited in domestic mail via air transportation but is permitted via surface transportation if the material can qualify as an ORM-D material and meet the following conditions as applicable:
a. The flashpoint is above 20°F (-7°C) but no more than 73°F (23°C); the liquid is in a metal primary receptacle not exceeding 1 quart, or in another type of primary receptacle not exceeding 1 pint, per mailpiece; enough cushioning surrounds the primary receptacle to absorb all potential leakage; the cushioning and primary receptacle are packed within a securely sealed secondary container that is placed within a strong outer shipping container; and each mailpiece is plainly and durably marked on the address side with “Surface Only” or “Surface Mail Only” and “ORM-D” immediately following or below the proper shipping name.
b. The flashpoint is above 73°F (23°C) but less than 100°F (38°C); the liquid is in a metal primary receptacle not exceeding 1 gallon, or in another type of primary receptacle not exceeding 1 quart, per mailpiece; enough cushioning surrounds the primary receptacle to absorb all potential leakage; the cushioning and primary receptacle are placed within a securely sealed secondary container that is placed within a strong outer shipping container; and each mailpiece is plainly and durably marked on the address side with “Surface Only” or “Surface Mail Only” and “ORM-D” immediately following or below the proper shipping name.
4.3Combustible Liquid Mailability
[6-12-03] Combustible liquid is prohibited in international mail. Combustible liquid is permitted in domestic mail if the material can qualify as an ORM-D material and meet the following conditions as applicable:
a. For surface transportation, if the flashpoint is 100°F (38°C) but no more than 141°F (60.5°C); the liquid is in a metal primary receptacle not exceeding 1 gallon, or in another type of primary receptacle not exceeding 1 quart, per mailpiece; enough cushioning surrounds the primary receptacle to absorb all potential leakage; the cushioning and primary receptacle are packed in a securely sealed secondary container that is placed within a strong outer shipping container; and each mailpiece is plainly and durably marked on the address side with “Surface Only” or “Surface Mail Only” and “ORM-D” immediately following or below the proper shipping name.
b. For surface or air transportation, if the flashpoint is above 141°F (60.5°C) but no more than 200°F (93°C); the liquid is in a primary receptacle not exceeding 1 gallon per mailpiece; enough cushioning surrounds the primary receptacle to absorb all potential leakage; the cushioning and primary receptacle are packed in a securely sealed secondary container that is placed within a strong outer shipping container; and each mailpiece is plainly and durably marked on the address side with “ORM-D” or “ORM-D AIR,” as applicable, immediately following or below the proper shipping name. Mailable material sent via surface transportation must be marked on the address side as “Surface Only” or “Surface Mail Only.” For air transportation, each mailpiece must bear a shipper’s declaration for dangerous goods.
c. For air or surface transportation, if the flashpoint is above 200°F (93°C) the material is not regulated as a hazardous material. Such nonregulated materials must be properly and securely packaged to prevent leakage under the general packaging requirements in C010.
4.4Cigarette Lighters
A cigarette lighter equipped with an ignition element and containing fuel is a Class 3 flammable liquid. A cigarette lighter that contains a flammable gas is classed as a Division 2.1 flammable gas. A cigarette lighter containing either flammable liquid or flammable gas is permitted only in domestic mail via surface transportation when all of the following conditions are met:
a. The design of the lighter and its packaging are approved by the DOT Associate Administrator for Hazardous Material Safety, per 49 CFR 173.21(i) and 173.308; and a DOT Approval Number (T-Number) is issued.
b. The prospective mailer of the lighter submits to the appropriate RCSC manager a written request for authorization to mail the lighter, accompanied by a legible photocopy of the official DOT notice conveying the approval described in 4.4a and a specimen of the actual lighter, the packaging materials in which each lighter is to be mailed, the number of mailpieces and mailing location; and the mailer receives from the RCSC manager a letter approving the requested authorization for mailing.
c. [6-12-03] When presented for mailing, the address side of the mailpiece containing the lighter prominently displays the T-Number, the proper shipping name “Lighter for Cigarette,” and the marking “Surface Only” or “Surface Mail Only”; all preparation and packaging requirements in the RCSC manager's approval letter are met; and a legible photocopy of the RCSC manager's approval letter accompanies the mailing.
5.0 Flammable Solids (Hazard Class 4)
5.1Definitions
Hazard class 4 consists of three divisions:
a. Division 4.1, Flammable Solids. Any solid material other than one classed as an explosive that, under conditions normally incident to transportation, is likely to cause fires through friction or retained heat from manufacturing or processing, or that can be ignited readily and, when ignited, burns so vigorously and persistently as to create a serious transportation hazard.
b. Division 4.2, Spontaneously Combustible. A liquid or solid pyrophoric material that even in small amounts and without an external ignition source can ignite within 5 minutes after coming in contact with air, or a self-heating material that, when in contact with air and without an energy supply, is liable to self-heat.
c. Division 4.3, Dangerous When Wet. A material that, by contact with water, is likely to become spontaneously flammable or to give off flammable or toxic gas at a rate greater than 1 liter per kilogram of the material per hour.
5.2Mailability
[6-12-03] Flammable solids are prohibited in international mail. Flammable solids are prohibited in domestic mail via air transportation. A flammable solid that can qualify as an ORM-D material is permitted in domestic mail via surface transportation if the material is contained in a secure primary receptacle having a weight of 1 pound or less; the primary receptacle(s) is packed in a strong outer shipping container with a total weight of 25 pounds or less per mailpiece; and each mailpiece is plainly and durably marked on the address side with “Surface Only” or “Surface Mail Only” and “ORM-D” immediately following or below the proper shipping name.
5.3Matches
[6-12-03] Matches are classified as flammable solids. Strike-anywhere matches are prohibited in international and domestic mail. Safety matches (book, card, or strike-on-box) are prohibited in international mail, and in domestic mail via air transportation, but are permitted in domestic mail via surface transportation if:
a. They do not ignite spontaneously under conditions normally incident to transportation or when subjected for 8 consecutive hours to a temperature of 220°F (93°C).
b. They cannot be readily ignited by friction unless struck on their own or a similar box, card, or book.
c. They are tightly packed in a securely sealed primary receptacle to prevent any shifting or movement that could cause accidental ignition by rubbing against adjoining items. The primary receptacle(s) is placed securely within an outer shipping container made of fiberboard, wood, or other equivalent material. Multiple primary receptacles may be placed in a single outer shipping container. The address side of the mailpiece must be marked “Surface Only” or “Surface Mail Only” and “Book Matches,” ”Strike-on-Card Matches,” or “Card Matches,” as appropriate. A shipping paper is not required.
d. The gross weight of each mailpiece is not more than 25 pounds.
6.0 Oxidizing Substances, Organic Peroxides (Hazard Class 5)
6.1Definition
Hazard class 5 consists of two divisions:
a. Division 5.1, Oxidizing Substances. A material that may, generally by yielding oxygen, cause or enhance the combustion of other materials.
b. Division 5.2, Organic Peroxides. Any organic compound that contains oxygen in the bivalent structure and that may be considered a derivative of hydrogen peroxide, where one or more of the hydrogen atoms have been replaced by organic radicals.
6.2Mailability
[6-12-03] Oxidizing substances and organic peroxides are prohibited in international mail. For domestic mail, a material that can qualify as an ORM-D material is permitted via air or surface transportation. Liquid materials must be enclosed within a primary receptacle having a capacity of 1 pint or less; the primary receptacle(s) must be surrounded by absorbent cushioning material and held within a leak-resistant secondary container that is packed within a strong outer shipping container. Solid materials must be contained within a primary receptacle having a weight capacity of 1 pound or less; the primary receptacle(s) must be surrounded with cushioning material and packed within a strong outer shipping container. Each mailpiece may not exceed a total weight of 25 pounds. The address side of each mailpiece must be plainly and durably marked with “ORM-D AIR” or “ORM-D,” as applicable, immediately following or below the proper shipping name. A mailable Class 5 material sent via surface transportation must be marked “Surface Mail” or “Surface Mail Only” on the address side. A mailable material sent via air transportation must bear a shipper’s declaration for dangerous goods.
7.0 Toxic Substances (Hazard Class 6, Division 6.1)
7.1Definitions
The terms used in the standards for Division 6.1 material are:
a. Toxic substance is a poisonous material, other than a gas, that is known to be so toxic to humans as to cause death, injury, or harm to human health if swallowed, inhaled, or contacted by the skin.
b. Oral toxicity applies to a liquid with a lethal dose (LD50) for acute oral toxicity of not more than 500 mg/kg or a solid with an LD50 for acute oral toxicity of not more than 200 mg/kg that when administered by mouth is likely to cause death within 14 days in half of the test animals.
c. Dermal toxicity applies to a material with an LD50 for acute dermal toxicity of not more than 1,000 mg/kg that when administered by continuous contact with bare skin is likely to cause death within 14 days in half of the test animals.
d. Inhalation toxicity applies to a dust or mist with a lethal concentration (LC50) for acute inhalation toxicity of not more than 10 mg/L; or a saturated vapor concentration in air at 68°F (20°C) of more than one-fifth of the LC50 for acute toxicity on inhalation of vapors and with an LC50 for acute inhalation toxicity of vapors of not more than 5,000 ml/m3; that when administered by continuous inhalation for 1 hour is likely to cause death within 14 days in half of the test animals.
e. Irritating material is any liquid or solid substance (e.g., tear gas) that gives off intense fumes and causes extreme irritation and impairment to a person's ability to function.
7.2Mailability
Toxic substances or poisons are prohibited in international mail. For domestic mail, a Division 6.1 toxic substance or poison that can qualify as an ORM-D material is permitted when packaged under the applicable requirements in 7.4. Certain other poisonous materials are permitted to be mailed only between the authorized parties and under the conditions in 7.3.
7.3Authorized Parties
A Division 6.1 toxic substance having an LD50 for oral toxicity of greater than 5mg/kg but less than or equal to 50 mg/kg is mailable only if packaged under the applicable requirements in 7.4 and when sent between authorized parties and under specified conditions, as follows:
a. Toxic substances for scientific use (not outwardly or of their own force dangerous or injurious to life, health, or property) may be sent only between manufacturers, dealers, bona fide research or experimental scientific laboratories, and employees of federal, state, or local governments who have official use for such poisons and are designated by the agency head to receive or send such poisons. For air transportation, a shipper’s declaration for dangerous goods is required.
b. Poisonous drugs and medicines may be sent only from the manufacturer or dealer of the drugs and medicines to licensed physicians, surgeons, dentists, pharmacists, druggists, cosmetologists, barbers, and veterinarians (18 USC 1716).
7.4Packaging and Marking
[6-12-03] The following requirements must be met, as applicable:
a. A toxic substance that can qualify as an ORM-D material and does not exceed a total capacity of 8 ounces per mailpiece is permitted if: the material is held in a primary receptacle(s); enough cushioning material surrounds the primary receptacle to absorb all potential leakage; the cushioning and primary receptacle(s) are packed in another securely sealed secondary container that is placed within a strong outer shipping container. Each mailpiece must be plainly and durably marked on the address side with “ORM-D” or “ORM-D AIR,” as applicable, immediately following or below the proper shipping name. Mailable material sent via surface transportation must be marked on the address side as “Surface Only” or “Surface Mail Only.”
b. Other toxic substances and poisons are permitted to be sent between the authorized parties and under the conditions in 7.3 when they do not exceed 8 ounces per mailpiece and if: the material is held in a leak-resistant primary receptacle(s); sufficient absorbent and cushioning material completely surround each primary receptacle; the primary receptacle(s) and the absorbent and cushioning materials are firmly held within a leakproof (for liquids) or siftproof (for solids) secondary container; the secondary container is firmly and securely held within a strong outer shipping container of 200-pound grade corrugated fiberboard or equivalent strength. The address side of each mailpiece must be marked with the proper shipping name and UN (or NA) identification number of the material (unless exempted by C024.11.2). Mailable materials sent via surface transportation must be marked on the address side as “Surface Only” or “Surface Mail Only.” Each mailpiece must bear a shipping paper.
7.5Irritating Material
Irritants are prohibited in international mail and domestic mail.
8.0 [6-12-03] Infectious Substances (Hazard CLass 6, Division 6.2)
8.1General
The materials covered under Division 6.2 include infectious substances (i.e., etiologic agents), biological products, cultures and stocks, diagnostic (clinical) specimens, regulated medical waste, sharps waste, toxins, and used health care products. Division 6.2 materials are not permitted in international mail or domestic mail, except when they are intended for medical or veterinary use, research, or laboratory certification related to the public health; and only when such materials are properly prepared for mailing to withstand shocks, pressure changes, and other conditions related to ordinary handling in transit. Mailable Division 6.2 materials sent as international mail must meet the standards in International Mail Manual 135. For domestic mail, mailable Division 6.2 materials must meet the applicable standards in 8.0. Unless otherwise noted, all mailable Division 6.2 materials in Risk Groups 2, 3, or 4 must be prepared to meet the requirements for air transportation.
8.2Definitions
The terms used in the standards for Division 6.2 materials are defined as follows:
a. Division 6.2 (infectious substance) means a material known to contain or suspected of containing a pathogen. A pathogen is a virus or microorganism (including its viruses, plasmids, or other genetic elements, if any) or a proteinaceous infectious particle (prion) that has the potential to cause disease in humans or animals. A Division 6.2 material must be assigned to a risk group as defined in 8.2f. Assignment to a risk group is based on the known medical condition and history of the source patient or animal, endemic local conditions, symptoms of the source patient or animal, or professional judgment concerning individual circumstances of the source patient or animal. Infectious substances are subject to applicable requirements in 42 CFR 72 (Interstate Shipment of Etiologic Agents).
b. Biological product means a virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood component or derivative, allergenic product, or analogous product used in the prevention, diagnosis, treatment, or cure of diseases in humans or animals. A biological product includes a material manufactured and distributed in accordance with one of the following provisions: 9 CFR 102 (Licenses for Biological Products); 9 CFR 103 (Experimental Products, Distribution, and Evaluation of Biological Products Prior to Licensing); 9 CFR 104 (Permits for Biological Products); 21 CFR 312 (Investigational New Drug Application); 21 CFR 314 (Applications for FDA Approval to Market a New Drug); 21 CFR 600–680 (Biologics); or 21 CFR 812 (Investigational Device Exemptions). A biological product known to contain or suspected of containing a pathogen in Risk Group 2, 3, or 4 must be classed as Division 6.2, described as an infectious substance, and assigned to UN 2814 or UN 2900, as appropriate, unless otherwise excepted by standard.
c. Cultures and stocks means a material prepared and maintained for growth and storage and containing a Risk Group 2, 3, or 4 infectious substance.
d. Diagnostic (clinical) specimen means any human or animal material, including excreta, secreta, blood and its components, tissue, and tissue fluids being transported for diagnostic or investigational purposes, but excluding live infected animals. A diagnostic specimen is not assigned a UN identification number unless the source patient or animal has or may have a serious human or animal disease from a Risk Group 4 pathogen, in which case it must be classed as Division 6.2, described as an infectious substance, and assigned to UN 2814 or UN 2900, as appropriate. Assignment to UN 2814 or UN 2900 is based on known medical condition and history of the patient or animal, endemic local conditions, symptoms of the source patient or animal, or professional judgment concerning individual circumstances of the source patient or animal.
e. Regulated medical waste, for USPS purposes, means a soft waste material (other than a sharp) known to contain or suspected of containing an infectious substance in Risk Group 2 or 3 and generated in the diagnosis, treatment, or immunization of human beings or animals; research on the diagnosis, treatment, or immunization of human beings or animals; or the production or testing of biological products. Soft medical waste includes items such as used rubber gloves, swabs, gauze, tongue depressors, etc. Regulated medical waste classified in Risk Group 4 is nonmailable.
f. Risk group means a ranking of a microorganism's ability to cause injury through disease. A risk group is defined by criteria developed by the World Health Organization (WHO) that are based on the severity of the disease caused by the organism, the mode and relative ease of transmission, the degree of risk to both an individual and a community, and the reversibility of the disease through the availability of known and effective preventive agents and treatment. There is no relationship between a risk group and a DOT packing group. Assignment to a risk group is based on the known medical condition and history of the source patient or animal, endemic local conditions, symptoms of the source patient or animal, or professional judgment concerning individual circumstances of the source patient or animal. The sender is responsible for accurately ranking a mailable material within the correct risk group. Exhibit 8.2f details the criteria for each risk group according to the level of risk.
Exhibit 8.2fRisk Group Criteria
| Risk Group | Pathogen | Risk to Individuals | Risk to Community |
|---|---|---|---|
| 4 | A pathogen that usually causes serious human or animal disease and that can be readily transmitted from one individual to another, directly or indirectly, and for which effective treatments and preventive measures are not usually available. | High | High |
| 3 | A pathogen that usually causes serious human or animal disease but does not ordinarily spread from one infected individual to another, and for which effective treatments and preventive measures are available. | High | Low |
| 2 | A pathogen that can cause human or animal disease but is unlikely to be a serious hazard, and, while capable of causing serious infection on exposure, for which there are effective treatments and preventive measures available and the risk of spread of infection is limited. | Moderate | Low |
| 1 | A microorganism that is unlikely to cause human or animal disease. A material containing only such microorganisms is not subject to regulation as a hazardous material, but it is subject to the packaging requirements in 8.10, unless otherwise noted in 8.0. | None or Very Low | None or Very Low |
g. Sharps, for USPS purposes, means any object contaminated with a pathogen or that may become contaminated with a pathogen through handling or during transportation and that is also capable of cutting or penetrating skin or a packaging material. Sharps include used medical waste such as needles, syringes, scalpels, broken glass, culture slides, culture dishes, broken capillary tubes, broken rigid plastic, and exposed ends of dental wires. Sharps waste classified in Risk Group 4 is nonmailable.
h. Toxin means a Division 6.1 material from a plant, animal, or bacterial source. A toxin containing an infectious substance or a toxin contained in an infectious substance must be classed as Division 6.2, described as an infectious substance, and assigned to UN 2814 or UN 2900, as appropriate.
i. Used health care product means a medical, diagnostic, or research device or piece of equipment, or a personal care product used by consumers, medical professionals, or pharmaceutical providers that does not meet the definition of a diagnostic specimen, biological product, regulated medical waste, or sharps waste, is contaminated with potentially infectious body fluids or materials, and is not decontaminated or disinfected to remove or mitigate the infectious hazard prior to transportation. A used health care product classified in Risk Group 4 is nonmailable.
8.3Nonregulated Materials
The following materials are not subject to regulation as Division 6.2 hazardous materials and are mailable when the packaging requirements in 8.10 are met:
a. A diagnostic (clinical) specimen known to contain or suspected of containing a microorganism in Risk Group 1, or that does not contain a pathogen. Also, a diagnostic specimen in which the pathogen has been neutralized or inactivated so that exposure to it cannot cause disease.
b. A biological product known to contain or suspected of containing a microorganism in Risk Group 1, or that does not contain a pathogen. Also any biological product, including an experimental product or component of a product, subject to Federal approval, permit, or licensing requirements, such as those required by the Food and Drug Administration (FDA) of the Department of Health and Human Services (HHS) or the U.S. Department of Agriculture (USDA).
c. Blood collected for blood transfusion or the preparation of blood products; blood products; tissues intended for use in surgical procedures; and human cell, tissues, and cellular and tissue-based products regulated under authority of the Public Health Service Act and/or the Food, Drug, and Cosmetic Act. Also, blood collected for blood transfusion or the preparation of blood products and sent for testing as part of the collection process, except where the person collecting the blood has reason to believe it contains a pathogen in Risk Group 2 or 3, in which case the test sample must be packaged under 8.6.
d. A material, including a Division 6.2 waste, that previously contained an infectious substance that has been treated by steam sterilization, chemical disinfection, or other appropriate method, so it no longer meets the definition of an infectious substance in Risk Group 2, 3, or 4.
e. Forensic material in Risk Group 1 transported on behalf of a U.S. government, state, local, or Indian tribal government agency.
f. Environmental microbiological samples, such as samples of dust from a ventilation system or mold from a wallboard, collected to evaluate occupational and residential exposure risks.
8.4Packaging—General
All materials mailable under the provisions in 8.0 must be properly packaged. Exhibit 8.4 lists the specific reference in 8.0 under which each type of mailable material must be packaged.
Exhibit 8.4Packaging References for Materials
Mailable Under 8.0
| Material | Risk Group | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Blood for Transfusion | 8.10 | 8.6 | 8.6 | nm |
| Biological Product | 8.10 | 8.5 | 8.5 | 8.5 |
| Culture or Stock | 8.10 | 8.5 | 8.5 | 8.5 |
| Diagnostic Specimen | 8.10 | 8.6 | 8.6 | 8.5 |
| Division 6.2 (Infectious Substance) | 8.10 | 8.5 | 8.5 | 8.5 |
| Forensic Material | 8.10 | 8.9 | 8.9 | 8.5 |
| Regulated Medical Waste | 8.7 | 8.7 | 8.7 | nm |
| Sharps Waste | 8.7 | 8.7 | 8.7 | nm |
| Toxin (Division 6.2) | 8.10 | 8.5 | 8.5 | 8.5 |
| Treated Medical Waste | 8.10 | n/a | n/a | n/a |
| Used Health Care Product | 8.8 | 8.8 | 8.8 | nm |
nm - nonmailable; n/a - not applicable
8.5Packaging of
Division 6.2 Infectious Substances
Division 6.2 materials include infectious substances (etiologic agents), biological products, cultures or stocks, and toxins known or suspected to contain a Risk Group 2, 3, or 4 pathogen. Division 6.2 also includes diagnostic specimens known or suspected to contain a Risk Group 4 pathogen. The packaging of Division 6.2 infectious substances is subject to these standards:
a. All Division 6.2 materials must meet the packaging requirements in 49 CFR 173.196. Either the primary receptacle or the secondary container must be capable of withstanding, without leakage, an internal pressure that produces a pressure differential of not less than 0.95 bar, 14 psi (95 kPa), and temperatures in the range of -40°F to 131°F (-40°C to 55°C) as required by 49 CFR 173.196.
b. The material must be packaged in a securely sealed and watertight primary receptacle (test tube, vial, etc.) that is enclosed in another watertight and durable secondary container that is securely sealed. Several primary receptacles may be enclosed in the secondary container if there is adequate cushioning material between them to prevent breakage during normal handling, and if the total volume of the material in all enclosed primary receptacles does not exceed 50 ml for liquids or 50 g for solids. The primary receptacle(s) and the secondary container must be marked with the international biohazard symbol as shown in Exhibit 8.7c(2).
c. The space between the primary receptacle(s) and the secondary container at the top, bottom, and sides must contain enough absorbent material to take up the entire contents of the primary receptacle(s) in case of breakage or leakage.
d. The primary receptacle(s) and the secondary container must be securely enclosed in an outer shipping container constructed of fiberboard or other equivalent material. No external surface of the outer shipping container may be less than 3.9 inches (100 mm) as required by 49 CFR 173.196. An itemized list of the contents of the primary receptacle(s) must be enclosed between the secondary container and the outer shipping container.
e. Each mailpiece must be designed and constructed so that, if it were subject to the environmental and test conditions in 49 CFR 178.609, there would be no release of the contents to the environment and no significant reduction in the effectiveness of the packaging.
f. All mailpieces sent under 8.5 must be sent First-Class Mail or Priority Mail and must be marked on the address side with the proper shipping name and UN number of the material (e.g., “UN 2814, Infectious Substances, Affecting Humans” or “UN 2900, Infectious Substances, Affecting Animals”). Each mailpiece must bear a DOT Class 6 label for infectious substances (etiologic agents), proper UN package specification markings, and orientation markings. A shipping paper is required. Any mailpiece classified as a Risk Group 4 material and that contains any of the select agents or toxins listed in 42 CFR 73.4 or 73.5 must meet all requirements in 42 CFR 72 and must also be sent using Registered Mail service.
g. Articles that include dry ice as a refrigerant for the infectious substance must meet the requirements in 49 CFR 173.196(b)(2)(ii).
8.6Packaging for Diagnostic Specimens
in Risk Group 2 or 3
A diagnostic (clinical) specimen known or suspected to contain a Risk Group 4 pathogen must be packaged under 8.5. A diagnostic specimen classified in Risk Group 1 must be packaged under 8.10. A diagnostic specimen classified in Risk Group 2 or 3 and that meets the definition in 8.2d must be sent as First-Class Mail, Priority Mail, or Express Mail. Such materials must be packaged in a triple packaging, consisting of a primary receptacle, secondary container, and outer shipping container, subject to the following specific requirements:
a. Liquid Diagnostic (Clinical) Specimens.
(1) The specimen must be contained in a leakproof and
securely sealed primary receptacle. A single primary receptacle may
not contain more than 500 ml of a specimen. Multiple primary receptacles
are permitted in a single mailpiece if the mailpiece does not contain
more than
4,000 ml. The primary receptacle(s) must be surrounded with sufficient
cushioning material to withstand shock and pressure changes and with
absorbent material capable of taking up the entire liquid contents should
the primary receptacle(s) leak.
(2) The primary receptacle(s) and the absorbent material must be securely packed within a secondary container in such a way that, under normal conditions of transport, the primary receptacle cannot break, be punctured, or leak its contents into the secondary container.
(3) The secondary container must be leakproof, securely sealed, and placed within a strong outer shipping container having suitable cushioning material such that any leakage of the contents does not impair the protective properties of the cushioning material or the outer shipping container. The secondary container must be marked with the international biohazard symbol as shown in Exhibit 8.7c(2).
(4) The primary receptacle(s) or the secondary container must be capable of withstanding, without leakage, an internal pressure producing a pressure differential of not less than 0.95 bar, 14 psi (95 kPA). The completed mailpiece must be capable of successfully passing the drop test in 49 CFR 178.603 at a drop height of at least 1.2 meters (3.9 feet). The address side of the outer shipping container must be clearly and durably marked “Diagnostic Specimen.” A shipping paper is not required.
b. Solid (or Dried) Diagnostic Specimens.
(1) The primary receptacle must be siftproof with a capacity of not more than 500 g (1.1 pounds).
(2) If several fragile primary receptacles are placed in a single secondary container, they must be individually wrapped or separated with sufficient cushioning material to prevent contact between them. The secondary container must be siftproof to contain the contents should the primary receptacle(s) leak. The secondary container must be marked with the international biohazard symbol as shown in Exhibit 8.7c(2).
(3) The outer shipping container may not exceed 4 kg (8.8 pounds) capacity. The outer shipping container must be clearly and durably marked “Diagnostic Specimen.” A shipping paper is not required.
8.7Sharps Waste and Other Mailable Regulated
Medical Waste
Regulated medical waste and sharps waste known to contain or suspected of containing an infectious substance in Risk Group 4 are nonmailable. Regulated medical waste and sharps waste as defined in 8.2e and 8.2g, respectively, and classified in Risk Group 1, 2, or 3 are permitted for mailing only using merchandise return service (see S923) with First-Class Mail or Priority Mail, subject to the following requirements:
a. Authorization. Each distributor or manufacturer of a complete regulated medical waste or sharps waste mailing container system (including all component parts required to safely mail such waste to a storage or disposal facility) must obtain authorization from the USPS prior to mailing. Before applying for authorization, each type of mailing container system must be tested and certified under the standards in 8.7d by an independent testing facility. The manufacturer or distributor in whose name the authorization is being sought must submit a written request to the manager, Mailing Standards, USPS Headquarters (see G043 for address). The request for authorization must contain the following:
(1) An irrevocable $50,000 surety bond or letter of credit as proof of sufficient financial responsibility to cover disposal costs if the manufacturer (or distributor) ceases doing business before all its waste container systems are disposed of or to cover cleanup costs if spills occur while the containers are in USPS possession. The surety bond or letter of credit must be issued in the name of the manufacturer or distributor seeking the authorization and must name the USPS as the beneficiary or obligee, as appropriate.
(2) Address of the headquarters or general business office of the distributor or manufacturer seeking the authorization.
(3) Address of each disposal and storage site.
(4) List of all types of mailing container systems to be covered by the request, a complete sample of each mailing container system, and proof of package testing certifications performed by the independent testing facility that subjected the packaging materials to the testing requirements in 8.7d.
(5) Copy of the proposed waste manifest (i.e., shipping paper) to be used with each mailing container system.
(6) 24-hour toll free telephone number for emergencies.
(7) List of the types of waste to be mailed for disposal in each mailing container system.
(8) Copy of the merchandise return service label to be used with each mailing container system.
b. Packaging. Regulated medical waste and sharps waste in Risk Group 4 are nonmailable. A waste material treated by steam sterilization, chemical disinfection, or other appropriate method, so it no longer meets the definition of an infectious substance in Risk Group 2, 3, or 4, must be packaged under 8.10. The packaging for regulated medical waste and sharps waste in Risk Group 1, 2, or 3 is subject to these standards:
(1) Regulated medical waste and sharps waste meeting the definitions in 8.2e and 8.2g, respectively, must be collected in a rigid, securely sealed, and leakproof primary receptacle. For sharps waste, the primary receptacle must also be puncture-resistant and may not have a maximum capacity that exceeds 3 gallons in volume. For regulated medical waste, the primary receptacle may not have a maximum capacity that exceeds 5 gallons in volume. Each primary receptacle may not contain more than 50 ml (1.66 ounces) of residual waste liquid. Each primary receptacle must display the international biohazard symbol shown in Exhibit 8.7c(2). Each primary receptacle must maintain its integrity when exposed to temperatures between 0° and 120°F.
(2) The primary receptacle must be packaged within a watertight secondary container or containment system. The secondary container may consist of more than one component. If one of the components is a plastic bag, it must be at least 3 mil in thickness and be used in conjunction with a strong fiberboard box. A plastic bag by itself does not meet the requirement for a secondary container. Several primary receptacles may be enclosed in a secondary container. The primary receptacle(s) must fit securely and snugly within the secondary container to prevent breakage during ordinary processing.
(3) The secondary container must be enclosed in a strong outer shipping container constructed of 200-pound grade corrugated fiberboard. The joints and flaps of the outer shipping container must be securely taped, glued, or stitched to maintain the integrity of the container. When tape or glue is used to secure an outer shipping container, the material must be water-resistant. Fiberboard boxes with interlock bottom flaps (i.e., easy-fold) are not permitted as outer shipping containers unless reinforced with water-resistant tape. The secondary container must fit securely and snugly within the outer shipping container to prevent breakage during ordinary processing.
(4) There must be enough material within a watertight barrier to absorb and retain three times the total liquid allowed within the primary receptacle (150 ml per primary receptacle) in case of leakage.
(5) Each mailpiece must not weigh more than 25 pounds.
(6) In each mailing container system, the authorized manufacturer or distributor must include a step-by-step instruction sheet that clearly details the proper sequence and method of container system assembly prior to mailing to prevent package failure during transport due to improper assembly. The instruction sheet must also include a customer service telephone number, or provide specific information on where such a telephone number is located elsewhere on the container system, for third-party end users to contact if they have assembly questions or find a component part is missing.
c. Mailpiece Labeling, Marking, and Documentation. Regulated medical waste and sharps waste must meet the following requirements:
(1) Each primary receptacle and outer shipping container must bear a label, which cannot be detached intact, showing: (a) the company name of the manufacturer or the distributor to which the mailing authorization is issued; (b) the USPS Authorization Number, and; (c) the container ID number (or unique model number) signifying that the packaging material is certified and that the manufacturer or distributor obtained the authorization required by 8.7a.
(2) The primary receptacle(s) and the outer shipping container must bear the international biohazard symbol in black with either a fluorescent orange or fluorescent red background as shown in Exhibit 8.7c(2).
Exhibit 8.7c(2)International
Biohazard Symbol
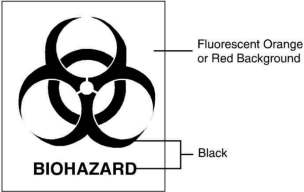
(3) Each mailpiece must have a four-part waste manifest, which also serves as the shipping paper. The manifest must be affixed to the outside of the mailpiece in an envelope or similar carrier that can be easily opened and resealed to allow review of the document. The manifest must comply with all applicable requirements imposed by the laws of the state from which the container system is mailed. At a minimum, the information in Exhibit 8.7c(3) must be on the manifest.
Exhibit 8.7c(3)Manifest for Regulated Medical Waste
and Sharps Waste Containers
| Manifest for Regulated Medical Waste and Sharps Waste Containers |
|---|
| 1. Generator (Mailer) |
| a. Name. b. Complete address (not a Post Office box). c. Telephone number. d. Description of contents of mailing container. “Regulated Medical Waste” or “Regulated Medical Waste–Sharps” is required as appropriate. e. Date container was mailed. f. State permit number of approved facility in which contents are to be disposed of. |
| 2. Destination Facility (Disposal Site) |
| Complete address (not a Post Office box). |
| 3. Generator’s (Mailer’s) Certification |
| The following certification statement must be printed on manifest: “I certify that this container has been approved for the mailing of [insert either “regulated medical waste” or “sharps waste,” as appropriate], has been prepared for mailing in accordance with the directions for that purpose, and does not contain excess liquid or nonmailable material in violation of the applicable Postal Service regulations. I AM AWARE THAT FULL RESPONSIBILITY RESTS WITH THE GENERATOR (MAILER) FOR ANY VIOLATION OF 18 USC 1716 WHICH MAY RESULT FROM PLACING IMPROPERLY PACKAGED ITEMS IN THE MAIL. I also certify that the contents of this consignment are fully and accurately described above by proper shipping name and are classified, packed, marked, and labeled, and in proper condition for carriage by air according to the national governmental regulations.” This statement must be followed by printed or typewritten name of generator (mailer), signature of generator, and date signed. |
| 4. Destination Facility (Storage or Disposal Site) |
| The following certification statement of receipt, treatment, and disposal must be printed on manifest: “I certify that the contents of this container have been received, treated, and disposed of in accordance with all local, state, and federal regulations.” This statement must be followed by printed or typewritten name of an authorized recipient at destination facility, signature of authorized recipient, and date signed. |
| 5. Transporter Intermediate Handler Other Than the Postal Service (If Different From Destination Facility) |
| a. Name. b. Complete address (not a Post Office box). c. Printed or typewritten name of transporter or intermediate handler. d. Signature of transporter or intermediate handler and date signed. |
| 6. Serialized Waste Manifests |
| Each waste manifest or mail disposal service shipping record must be serialized using a unique numbering system for identification purposes. |
| 7. Comment Area |
| Each manifest must contain an area designated for entering comments or noting discrepancies. |
| 8. Completion and Distribution of Waste Manifest |
| Each manifest must contain instructions for properly completing the four-part form. Copies of the form must be distributed as follows: a. One copy must be kept by generator (mailer). b. One copy must be kept by transporter or intermediate handler for 90 days. c. One copy must be kept by destination facility for 90 days. d. One copy must be mailed to generator by destination facility. |
| 9. Emergency Telephone Number |
| Each manifest must bear the following statement with appropriate information: “IN CASE OF EMERGENCY, OR THE DISCOVERY OF DAMAGE OR LEAKAGE, CALL 1-800-###-####.” |
(4) The outer shipping container must bear a properly prepared merchandise return service label (see S923). The merchandise return service permit must be held in the same name as that of the authorized medical waste manufacturer or distributor.
(5) The outer shipping container must be marked on two opposite side walls with the package orientation marking in 49 CFR 173.312 to identify the proper upright position of the mailpiece during handling.
(6) Mailpieces containing regulated medical waste or sharps waste must be marked on the address side with the correct UN number and proper shipping name (e.g., “Regulated Medical Waste, UN 3291” or “Regulated Medical Waste–Sharps, UN 3291”).
d. Package Testing. Testing must be performed by an independent testing facility on one sample of each type of mailing container system to prove compliance with 8.7a. The sample mailing container system must withstand the tests in 49 CFR 178.604 (leakproof test), 178.606 (stacking test), 178.608 (vibration standard), and 178.609(e), (f), and (h) (test requirements for packaging for infectious substances). In addition, the absorbent material must withstand an absorbency test that satisfies the requirements in 8.7b(4). The test results must show that if every container system prepared for mailing were to be subject to the environmental and test conditions in 49 CFR, there would be no release of the contents to the environment and no significant reduction in the effectiveness of the packaging. Periodic retesting must be performed whenever a change is made to the design of the container system or every 24 months, whichever occurs first.
8.8Packaging of Used Health Care Products
A used health care product known or suspected to contain a Risk Group 4 pathogen is nonmailable. A used health care product meeting the definition in 8.2i, classified in Risk Group 1, 2, or 3, and being returned to the manufacturer or manufacturer's designee is mailable as First-Class Mail, Priority Mail, or Express Mail subject to the following packaging requirements:
a. Each used health care product must be drained of liquid to the extent possible and placed in a watertight primary receptacle designed and constructed to ensure that it remains intact under normal conditions of transport. For a used health care product capable of cutting or penetrating skin or packaging material, the primary receptacle must be capable of retaining the product without puncture of the packaging under normal conditions of transport. The primary receptacle must be marked with the international biohazard symbol as shown in Exhibit 8.7c(2).
b. Each primary receptacle must be placed inside a watertight secondary container designed and constructed to ensure that it remains intact under normal conditions of transport. The secondary container must also be marked with the international biohazard symbol as shown in Exhibit 8.7c(2).
c. The secondary container must be placed inside an outer shipping container with sufficient cushioning material to prevent movement between the secondary container and the outer shipping container. An itemized list of the contents of the primary receptacle and information concerning possible contamination with a Division 6.2 material, including its possible location on the product, must be placed between the secondary container and the outer shipping container. A shipping paper and a content marking on the outer shipping container are not required.
8.9Packaging of Forensic Material in
Risk Groups 2 and 3
Forensic material in Risk Group 1 sent on behalf of a U.S. government, state, local, or Indian tribal government agency must be packaged under 8.10. Forensic material known or suspected to contain a Risk Group 4 infectious substance must be packaged under 8.5. Forensic material known or suspected to contain a Risk Group 2 or 3 pathogen is mailable as First-Class Mail, Priority Mail, or Express Mail when packaged in a triple packaging consisting of a primary receptacle, secondary container, and outer shipping container as follows:
a. The forensic material must be held within a securely sealed primary receptacle. The primary receptacle must be surrounded by sufficient absorbent material (for liquids) and cushioning material to protect the primary container from breakage. The absorbent material must be capable of taking up the entire liquid contents of the primary receptacle in case of leakage. The primary receptacle must be marked with the international biohazard symbol as shown in Exhibit 8.7c(2).
b. The primary receptacle and the absorbent and cushioning material must be enclosed in a watertight and securely sealed secondary container. The secondary container must also display the international biohazard symbol as shown in Exhibit 8.7c(2).
c. The secondary container must be firmly and snugly packed within a strong outer shipping container that is securely sealed. A shipping paper and a content marking on the outer shipping container are not required.
8.10Packaging for Risk Group 1 Materials
Division 6.2 materials in Risk Group 1 are not subject to regulation as hazardous materials (see 8.3), but when presented for mailing they must be properly packaged. Regulated medical waste, sharps waste, and used health care products classified in Risk Group 1 must be packaged and mailed under the applicable requirements in 8.7 or 8.8. All other Risk Group 1 materials are mailable as First-Class Mail, Priority Mail, Express Mail, or Package Services. Such materials must be held within a securely sealed primary receptacle. The primary receptacle must be surrounded by sufficient absorbent material (for liquids) and cushioning material to protect the primary receptacle from breakage. The absorbent material must be capable of taking up the entire liquid contents of the primary receptacle in case of leakage. Either the primary receptacle or the inner packaging must be marked with the international biohazard symbol as shown in Exhibit 8.7c(2). The primary receptacle and the absorbent and cushioning material must be snugly enclosed in a strong outer shipping container that is securely sealed. A shipping paper and a content marking on the outer shipping container are not required. Risk Group 1 diagnostic specimens and biological products are subject to the following packaging standards:
a. Liquid Diagnostic (Clinical) Specimens and Biological Products. A diagnostic (clinical) specimen in Risk Group 4 or a biological product in Risk Group 2, 3, or 4 must be packaged under 8.5. A diagnostic specimen in Risk Group 2 or 3 must be packaged under 8.6. The packaging of a diagnostic specimen in Risk Group 1 (e.g., a urine specimen or blood specimen used in drug testing programs or for insurance purposes) or a biological product (e.g., polio vaccine) in Risk Group 1 is subject to the following standards:
(1) Not Exceeding 50 ml. A diagnostic specimen or biological product consisting of 50 ml or less per mailpiece must be packaged in a securely sealed primary receptacle. Two or more primary receptacles whose combined volume does not exceed 50 ml may be enclosed within a single mailpiece. Sufficient absorbent material and cushioning material to withstand shock and pressure changes must surround the primary receptacle(s), or be otherwise configured to take up the entire liquid contents in case of leakage. The primary receptacle(s) and the absorbent cushioning must be enclosed in a secondary container having a leakproof barrier that can prevent failure of the secondary container if the primary receptacle(s) should leak during transport. The secondary container must be securely sealed and it may serve as the outer shipping container provided it has sufficient strength to withstand ordinary postal processing. The secondary container must be marked with the international biohazard symbol as shown in Exhibit 8.7c(2), except when the secondary container also serves as the outer shipping container. In that case, the biohazard symbol must appear either on the inner packaging or on the primary container. A shipping paper and a content marking on the outer shipping container are not required.
(2) Exceeding 50 ml. A clinical specimen or biological product that exceeds 50 ml must be packaged in a securely sealed primary receptacle. A single primary receptacle must not contain more than 500 ml of specimen. Two or more primary receptacles whose combined volume does not exceed 500 ml may be enclosed in a single secondary container. Sufficient absorbent material and cushioning material to withstand shock and pressure changes must surround the primary receptacle(s), or be otherwise configured to take up the entire liquid contents in case of leakage. The primary receptacle(s) and the absorbent cushioning must be enclosed in a secondary container having a leakproof barrier that can prevent failure of the secondary container if the primary receptacle(s) should leak during transport. The secondary container cannot serve as the outer shipping container. The secondary container must be marked with the international biohazard symbol as shown in Exhibit 8.7c(2). The secondary container must be securely and snugly enclosed in a fiberboard box or container of equivalent strength that serves as the outer shipping container. The maximum amount of a specimen that may be enclosed in a single mailpiece must not exceed 4,000 ml. A shipping paper and a content marking on the outer shipping container are not required.
b. Solid (or Dried) Specimens. A solid or dry specimen, such as a saliva swab, blood spot, or fecal smear in Risk Group 1 must be completely dried prior to placing it in or on a secure primary receptacle. Cushioning material to withstand shock and pressure changes is required only if the dry specimen is held in a breakable primary receptacle. When required, the cushioning material must surround the primary receptacle to prevent breakage or damage to the primary receptacle. The primary receptacle (and cushioning material, if required) must be enclosed in a secondary container having a leakproof barrier that can prevent failure of the secondary container if the primary receptacle breaks during shipment. The secondary container must be securely sealed and it may serve as the outer shipping container provided it has sufficient strength to withstand ordinary postal processing. The secondary container must be marked with the international biohazard symbol as shown in Exhibit 8.7c(2), except when the secondary container also serves as the outer shipping container. In that case, the biohazard symbol must appear either on the inner packaging or on the primary container. A shipping paper and a content marking on the outer shipping container are not required.
9.0 Radioactive Materials (Hazard Class 7)
Radioactive materials are prohibited in international mail and domestic mail if required to bear the DOT Radioactive White-I, Radioactive Yellow-II, or Radioactive Yellow-III label (49 CFR 172.436, 172.438, or 172.440, respectively) or if it contains quantities of radioactive material in excess of those authorized in Publication 52, Hazardous, Restricted, or Perishable Mail. Radioactive materials are prohibited in domestic mail via air transportation. For international mail, the standards in IMM 135 apply.
10.0 Corrosives (Hazard Class 8)
10.1Definition
A corrosive is any liquid or solid that causes visible destruction or irreversible alteration in human skin tissue at the site of contact or a liquid that has a severe corrosion rate on steel.
10.2Mailability
[6-12-03] Corrosives are prohibited in international mail. A corrosive that can qualify as an ORM-D material is permitted in domestic mail via air or surface transportation subject to these limitations:
a. Liquid Corrosive. A liquid mixture must be 1 pint or less and must contain 15% or less corrosive material with the remainder of the mixture not being a hazardous material, unless otherwise specified for a specific corrosive material. Primary receptacles must be securely sealed compatible glass bottles that are enclosed within securely sealed metal or plastic secondary containers. The secondary container must be packed within a strong outer shipping container that does not exceed 25 pounds per mailpiece.
b. Solid Corrosive. A solid mixture must be 10 pounds or less per primary receptacle and must contain 10% or less corrosive material with the remainder of the mixture not being a hazardous material, unless otherwise specified for a specific corrosive solid. The primary receptacle(s) and secondary container must be securely sealed compatible siftproof containers packed in strong outer shipping container. The total weight of a mailable solid corrosive cannot exceed 25 pounds per mailpiece.
10.3Marking
[6-12-03] For surface transportation, the mailpiece must be plainly and durably marked on the address side with “Surface Only” or “Surface Mail Only” and “ORM-D” immediately following or below the proper shipping name. For air transportation, the mailpiece must be plainly and durably marked on the address side with “ORM-D AIR” immediately following or below the proper shipping name and must bear a shipper's declaration for dangerous goods.
10.4Nonspillable Wet Electric Storage
Batteries
[6-12-03] A battery containing liquid electrolyte is prohibited from mailing unless the battery casing is completely sealed to prevent the liquid corrosive from spilling during handling. Nonspillable batteries with UN2800 are prohibited in international mail, but may be sent as domestic mail via air or surface transportation under the following conditions:
a. The nonspillable battery must be protected from short circuits, surrounded with sufficient cushioning material, and securely packaged in a strong fiberboard box that serves as the outer shipping container.
b. The outer shipping container must be marked “NONSPILLABLE BATTERY, UN2800” on the address side.
c. The nonspillable battery must be capable of withstanding the vibration and pressure differential tests cited in 49 CFR 173.159(d)(i) and (ii).
d. Only one nonspillable battery is allowed per mailpiece and the weight of the mailpiece cannot exceed 25 pounds.
11.0 Miscellaneous Hazardous Materials (Hazard Class 9)
11.1Definition
[6-12-03] A miscellaneous hazardous material is a substance or article that presents a hazard during transportation but does not meet the definition of any other hazard class. Examples of miscellaneous hazardous materials (not all of which are mailable) include solid dry ice, elevated temperature substances, environmentally hazardous substances, life-saving appliances, and asbestos.
11.2Mailability
A miscellaneous hazardous material is prohibited in international mail. A miscellaneous hazardous material that can qualify as an ORM-D material is permitted for domestic mail via air or surface transportation, subject to the applicable 49 CFR requirements.
11.3Marking
[6-12-03] For surface transportation, the mailpiece must be plainly and durably marked on the address side with “Surface Only” or “Surface Mail Only” and “ORM-D” immediately following or below the proper shipping name. For air transportation, a mailable material must be plainly and durably marked on the address side with “ORM-D AIR” immediately following or below the proper shipping name and bear a shipper's declaration for dangerous goods.
11.4Dry Ice
[6-12-03] Dry ice (carbon dioxide solid) is prohibited in international mail. Dry ice is permitted in the domestic mail via air or surface transportation when used as a refrigerant to cool the contents of a mailpiece. A mailpiece containing dry ice must be packed in a container that is designed to permit the release of carbon dioxide gas and prevent a build-up of pressure that could rupture the parcel. Containers must conform to 49 CFR 173.217 and 175.10(a)(13). Additionally, the following applies:
a. Air Transportation Requirements. Each mailpiece may not contain more than 5 pounds of dry ice. The address side of each mailpiece must be clearly marked “Carbon Dioxide Solid, UN1845” or “Dry Ice, UN1845” along with the net weight of the dry ice and the identity of the contents being cooled. A shipper’s declaration prepared in triplicate and a DOT Class 9 warning label for miscellaneous hazardous materials must be affixed to the outside of the mailpiece.
b. Surface Transportation Requirements. The amount of dry ice per mailpiece may exceed 5 pounds. The address side of each mailpiece must be clearly marked “Carbon Dioxide Solid” or “Dry Ice” and “Surface Only” or “Surface Mail Only” along with the net weight of the dry ice and the identity of the contents being cooled. A shipper’s declaration and a DOT Class 9 warning label are not required for the dry ice.
12.0 OTHER REGULATED MATERIALS—Magnetized Materials
[6-12-03] A magnetized material is not classified within any of the nine hazard classes. Such material is regulated as a hazardous material only if offered for carriage on air transportation and when it has a magnetic field strength capable of causing the deviation of aircraft instruments. Regulated magnetized materials are mailable subject to the following limitations:
a. Definition. A magnetized material is any article that has a magnetic field strength capable of causing the deviation of aircraft instruments. A magnetized material is regulated as a hazardous material when it is presented for air transportation and has a measurable magnetic field strength greater than 0.00525 gauss at 15 feet. Magnetized materials include magnets and magnetized devices such as magnetrons and light meters of sufficient strength to possibly cause erroneous aircraft compass readings. If the maximum field strength observed at a distance of 7 feet is less than 0.002 gauss or there is no significant compass deflection (less than 0.5 degree), the article is not restricted as a magnetized material.
b. Mailability. Regulated magnetized material is prohibited in international mail. A material with a measurable magnetic field strength greater than 0.00525 gauss at 15 feet is prohibited from domestic mail via air transportation. Mailable materials must be packaged and marked as specified in Publication 52, Hazardous, Restricted, and Perishable Mail. Mailable material permitted via air transportation must bear a shipper’s declaration for dangerous goods. Magnetized material is not regulated as a hazardous material when transported via surface transportation.
DMM Issue 58 (8-10-03)